Autoclaves and High-Pressure Steam Sterilization: Principles, Types, and Industrial Applications
Posted by Admin | 01 Aug
What Is an Autoclave?
An autoclave is a pressure chamber used to sterilize equipment, materials, and waste by subjecting them to high-pressure saturated steam. It’s a cornerstone technology in medical, pharmaceutical, microbiological, and industrial settings for ensuring the elimination of all microbial life—including spores, which are notoriously resistant to chemical and dry heat methods.
How Does Steam Sterilization Work?
Steam sterilization operates on a principle of thermal destruction of microorganisms. Autoclaves increase pressure within a sealed chamber to raise the temperature of steam beyond its normal boiling point—usually between 121°C (250°F) and 134°C (273°F)—and maintain that condition for a specified time (typically 15 to 30 minutes depending on load type and density).
Steam penetrates porous materials and denatures proteins in microorganisms, effectively killing bacteria, viruses, fungi, and spores.
Key Components of an Autoclave:
Chamber: The pressure vessel where sterilization occurs.
Steam Generator: Supplies the necessary saturated steam.
Control Panel: Allows operators to set temperature, pressure, and cycle time.
Safety Valves & Interlocks: Prevent dangerous pressure buildup.
Vacuum System (in some models): Removes air to enable better steam penetration.
Types of Autoclaves:
Gravity Displacement Autoclave:
Steam displaces air naturally in the chamber.
Common in laboratories and clinics.
Pre-Vacuum (High-Vacuum) Autoclave:
A vacuum pump removes air before steam injection.
Ensures deep penetration in complex or wrapped instruments.
Widely used in hospitals and surgical centers.
Positive Pressure Displacement Autoclave:
Uses pressure differentials for steam circulation.
Suitable for large-scale industrial applications.
Double Door (Pass-Through) Autoclaves:
Used in cleanroom environments.
Minimizes cross-contamination by separating sterile and non-sterile zones.
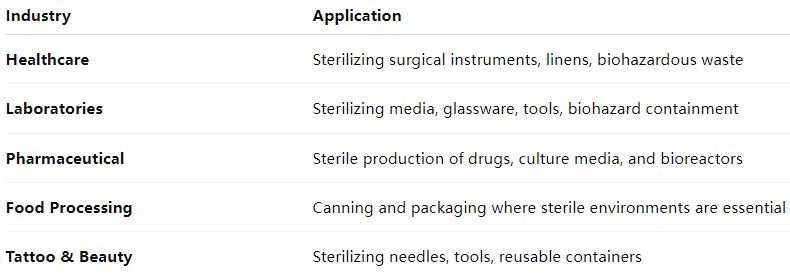
Applications Across Industries:
Advantages of Autoclave Sterilization:
Highly Effective: Destroys all microorganisms including spores.
Non-Toxic: Leaves no harmful residue.
Reliable: Time-tested across industries.
Cost-Efficient: Especially for reusable instruments and materials.
Limitations and Considerations:
Material Sensitivity: Not suitable for heat- or moisture-sensitive items (e.g., certain plastics or electronics).
Load Configuration: Poor loading can prevent proper steam penetration.
Cycle Validation Required: Especially in regulated environments like pharma or clinical labs.
Maintenance & Validation:
Routine checks for gaskets, pressure gauges, and steam traps.
Biological indicators (e.g., Geobacillus stearothermophilus spores) used to verify sterilization cycles.
Annual calibration and requalification recommended for compliance with standards like ISO 17665, EN 285, or FDA CFR 21 Part 11.
Emerging Trends:
Smart Autoclaves: Integrated with IoT for remote monitoring and analytics.
Energy Efficiency Improvements: Using advanced vacuum pumps and heat recovery systems.
Compact Models: Designed for point-of-use sterilization in field labs and mobile units.


 English
English русский
русский Français
Français Español
Español bahasa Indonesia
bahasa Indonesia Deutsch
Deutsch عربى
عربى 中文简体
中文简体


















