Bowie-Dick Test and Sterilization Protocols for Small Steam Sterilizers: Classification, Operation, and Verification Standards
Posted by Admin | 03 Jun
According to standards such as "Monitoring Methods and Evaluation Requirements for the Sterilization Effect of Small Steam Sterilizers" (GB/T 30690-2014), "Hospital Central Sterile Supply Department Part 3: Standards for Monitoring the Effectiveness of Cleaning, Disinfection and Sterilization" (WS 310.3-2016), and "Technical Specifications for Disinfection and Sterilization of Dental Instruments" (WS 506-2016), a small steam sterilizer refers to a steam sterilizer with a chamber volume not exceeding 60 liters.

Q: How does a small steam sterilizer complete a sterilization cycle?
Air Removal Phase
The air inside the sterilizer chamber is removed. Different types of small steam sterilizers use different air removal methods (such as vacuum extraction, pulsed steam displacement, or gravity displacement) to evacuate air from the chamber.
Heating Phase
After the air is removed, the exhaust valve closes, and steam continuously enters the sterilization chamber. This causes a rapid increase in temperature and pressure. The sterilized items are then penetrated by saturated steam and heated to the target temperature.
Sterilization Phase
Once the target temperature is reached, it is maintained for a specified period to complete sterilization (e.g., 121°C for 15 minutes or 132°C for 4 minutes).
Pressure Release Phase
After the designated sterilization time is completed, the exhaust valve opens, steam is released, and the pressure inside the chamber drops rapidly.
Drying Phase
Depending on the sterilization method, items are dried either through vacuum drying or pressure drying.
Air Intake Phase
After the sterilization cycle ends, sterilizers with B or S cycles draw filtered air from the environment into the chamber through an air filter, allowing the pressure to return to atmospheric levels.
Q: What sterilization cycles are available in small steam sterilizers?
B-type cycle
Suitable for sterilizing both wrapped and unwrapped loads, including solid, hollow, and porous items.
N-type cycle
Used only for sterilizing unwrapped solid loads.
S-type cycle
Designed for specific loads as defined by the manufacturer. These include unwrapped solid loads and at least one of the following: porous loads, small porous strips, hollow loads, single wrapped items, or multi-layer wrapped loads.
Q: What are the classifications and applications of small steam sterilizers?
1. Gravity Displacement Steam Sterilizer
(N-type sterilizer or sterilization cycle)
This type uses the principle of gravity displacement, where hot steam enters the sterilizer from the top and pushes the cold air downward and out through the bottom exhaust port. The expelled cold air is replaced by saturated steam, and the latent heat released by the steam sterilizes the items.
Applications: Suitable for sterilizing items that can withstand high temperature and humidity, such as microbial cultures, liquids, pharmaceuticals, laboratory waste, and non-porous items.
Limitations: Not suitable for sterilizing oils, powders, hollow items, surgical instruments, cavity instruments, or dental handpieces.
2. Pre-vacuum Steam Sterilizer
(B-type sterilizer or sterilization cycle)
This type uses mechanical vacuum to create negative pressure inside the chamber, allowing steam to rapidly penetrate the interior of the items. The latent heat of the steam ensures effective sterilization.
Applications: Ideal for sterilizing hollow instruments, porous items, and textiles that are heat- and moisture-resistant.
Limitations: Not suitable for liquids, oils, or powders.
3. Positive Pressure Pulse Displacement Steam Sterilizer
(S-type sterilizer or sterilization cycle)
This type uses the principle of pulsed steam displacement under positive pressure. Saturated steam is repeatedly pulsed into the chamber at a pressure above atmospheric level, pushing out cold air via pressure differences. The latent heat of the steam then sterilizes the items.
Applications: Suitable for solid items without lumens, and for certain specific lumens and porous items, provided their sterilization efficacy is verified through equivalent load testing.
Limitations: Not suitable for sterilizing textiles, medical waste, liquids, oils, or powders.
Note: When sterilizing dental instruments, the pre-vacuum steam sterilizer (B-type) is preferred. If using an S-type sterilizer, always follow the manufacturer’s specified scope of sterilization and ensure the items fall within that range.
Q: What are the validation principles for small steam sterilizers?
According to Clause 4.1 of GB/T 30690-2014: Monitoring Methods and Evaluation Requirements for the Sterilization Effect of Small Steam Sterilizers, sterilization parameters, sterilization effectiveness, and biological safety of the exhaust port must be validated annually.
Validation should be performed using load types corresponding to the sterilization cycle type:
B-type cycle: Use a corresponding lumen-type process challenge device (PCD) for validation.
N-type cycle: Use exposed solid items for validation.
S-type cycle: Select load types specified by the manufacturer and verify using matching test items.
Q: What are the sterilization parameters for a small pressure steam sterilizer?
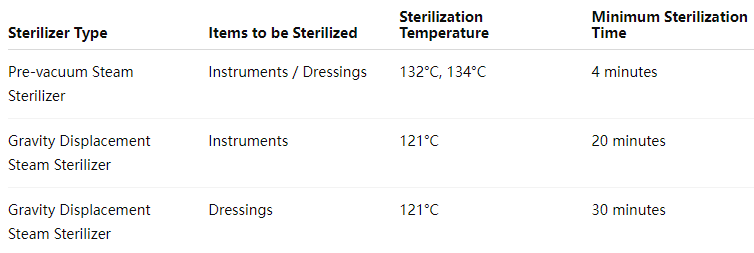
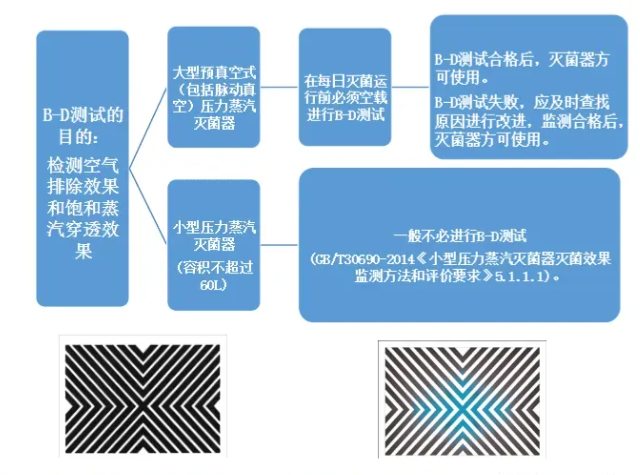
Q: Is it necessary to do a B-D test before the small pressure steam sterilizer is turned on every day?
The B-D test is applicable to pre-vacuum (including pulsating vacuum) sterilizers to monitor whether there is cold air remaining in the sterilizer. According to "Sterilization Effect Monitoring Method and Evaluation Requirements for Small Pressure Steam Sterilizers" (GB/T30690-2014) 5.1.1.1 Small pressure steam sterilizers generally do not need to perform B-D tests. If a B-D test is performed, it can be performed as follows: Under no-load conditions, place the B-D test object on the front bottom layer of the sterilizer, close to the cabinet door and exhaust port. There is nothing in the cabinet except the test object. After the B-D test cycle, take out the B-D test paper and observe the color change.
Q: Does the operation of a small pressure steam sterilizer require a work permit?
According to the management requirements of TSG21-2016 "Safety Technology of Fixed Pressure Vessels", a pressure steam sterilizer with a volume of ≥30 liters requires a special equipment operation permit to operate.
Q: How to monitor the sterilization effect of a small pressure steam sterilizer?
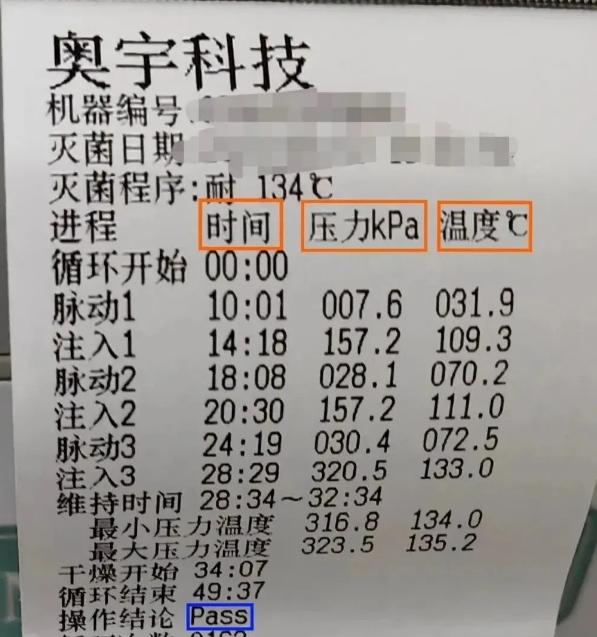
- Physical monitoring: Physical monitoring is also called process monitoring, which means that each sterilization should continuously monitor and record the sterilization parameters such as temperature, pressure and time during sterilization. The sterilization temperature should fluctuate within +3°C, and the time should meet the minimum sterilization time requirements. At the same time, the time, temperature and pressure values of all critical points should be recorded, and the results should meet the sterilization requirements. Frequency: It must be carried out for each sterilization cycle.
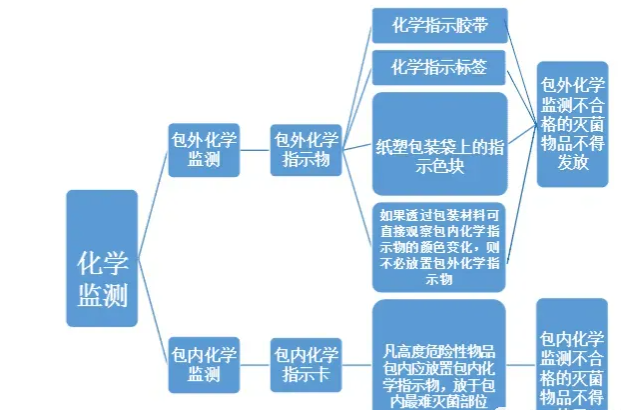
2. Chemical monitoring: The color change of the chemical indicator can be used to visually determine whether the requirements are met. Chemical monitoring can be divided into external indicators and internal indicators.
(1) External chemical monitoring: Use external chemical indicators, including chemical indicator tape, chemical indicator labels, and indicator color blocks on paper and plastic packaging bags. According to "Chemical indicator tape should be affixed to the surface of each item to be sterilized (except for packaging bags with chemical indicator color blocks)" 1. "If the color change of the chemical indicator in the package can be directly observed through the packaging material, there is no need to place an external chemical indicator" 2. When using paper and plastic packaging bags, there is no need to affix additional chemical indicators.
After a sterilization cycle, if the color block on the chemical indicator tape or paper and plastic bag changes color evenly and meets the standards specified by the manufacturer, it is qualified, indicating that the package has undergone the sterilization procedure, but it does not mean that the sterilization quality is qualified. If the color change does not meet the standard, the external chemical monitoring is unqualified, and sterilized items that fail the external chemical monitoring shall not be released.
(2) Internal chemical monitoring: Use the internal chemical indicator card. All highly dangerous items should be placed in the package with chemical indicators in the most difficult to sterilize part of the package (generally, the chemical indicator card is placed in the center of the package to be sterilized). If there is no item package, it is placed in the part of the sterilizer that is more difficult to sterilize (generally above the exhaust port). After a sterilization cycle, if the chemical indicator card in the package changes color and meets the standard, it means that the saturated steam has penetrated the sterilized items, the key parameters of sterilization (such as temperature and time) meet the standards, and the sterilization is qualified. If the color change does not meet the standards, the sterilization is unqualified. Sterilized items that fail the chemical monitoring in the package shall not be used. It should be noted that the qualified chemical monitoring in the package does not mean that the items are necessarily sterile. The sterility of the sterilized items needs to be confirmed by biological monitoring.
Frequency: It needs to be carried out in every sterilization cycle.
3. Biological monitoring: Cultivate the biological indicators that have gone through the sterilization cycle and the control biological indicators of the same batch that have not gone through the sterilization cycle at the same time. Judge the biological monitoring results by color comparison. For biological monitoring, you should choose a commonly used and representative sterilization package for the sterilizer to make a biological monitoring package, or use a biological PCD. Place it in the most difficult part of the sterilizer to sterilize (the closest to the exhaust port), and the sterilizer should be fully loaded.

Q: Is the frequency of biological monitoring of small high-pressure steam sterilizers one week or one month?
1. Appendix E of the Technical Operation Specifications for Sterilization of Oral Instruments (WS506-2016) states that small pressure sterilizers in use should be biologically monitored monthly.
2. 4.4.2.3 of the "Part 2 of the Hospital Sterilization Supply Center: Technical Operation Specifications for Cleaning, Disinfection and Sterilization" (WS 310.2-2016) states that biological monitoring of pressure sterilizers should be monitored at least once a week. Implants should be biologically monitored for each batch, and they can only be released after the biological monitoring is qualified.
3. "Principles of Clinical Laboratory Waste Treatment" (WS/T249-2005) 4.4.1 Pressure steam sterilization: Infectious laboratory waste, equipment and glassware can be decontaminated by pressure steam sterilization. Biological indicators (such as Bacillus stearothermophilus spores) should be used at least once a month to monitor the treatment effect. The treatment process should be carried out at 121℃ (the center temperature of the treated object is not less than 115℃), and the time is 60 to 90 minutes (not less than 20 minutes).
4. "Expert Consensus on Basic Requirements for Construction of Clinical Microbiology Laboratories": "Pressure sterilizers should use chemical indicators every time and biological indicators every week to monitor the sterilization effect. The accuracy of the pressure gauge, caliper, thermometer, hygrometer, and pipette of the pressure sterilizer should be verified, calibrated or calibrated regularly."
5. "Sterilization Effect Monitoring Method and Evaluation Requirements for Small Pressure Steam Sterilizers" (GB/T 30690-2014) Requirements for the frequency of biological monitoring of small pressure steam sterilizers: should be determined according to the nature of the sterilization object, and can be implemented in accordance with relevant standards and specifications.
According to the above provisions, the frequency of biological monitoring of small pressure steam sterilizers should be determined according to the sterilization object. If there are clear standards and specifications, they should be implemented according to the specifications. If there are no clear standards and specifications, it is recommended to be strict rather than lenient, and biological monitoring should be carried out once a week. However, it should be clear that large high-pressure steam sterilizers (more than 60 liters) must be biologically monitored once a week.


 English
English русский
русский Français
Français Español
Español bahasa Indonesia
bahasa Indonesia Deutsch
Deutsch عربى
عربى 中文简体
中文简体


















