Dental Autoclave for Clinics: Load-Driven Selection, Monitoring & Audit-Ready SOP
Posted by Admin | 15 Dec

1) The only selection rule that matters: your load determines the autoclave
Dental practices rarely process “instruments” as a single category. What you actually sterilize is a mix of unwrapped solids, wrapped/pouched packs for storage, hollow/lumen items, and mixed loads.
The failure mechanism that drives selection is simple: air retention prevents saturated steam contact, especially in wrapped packs and hollow/lumen items. That’s why air removal performance (pre-vacuum) and drying stability become decisive in dental settings. Source
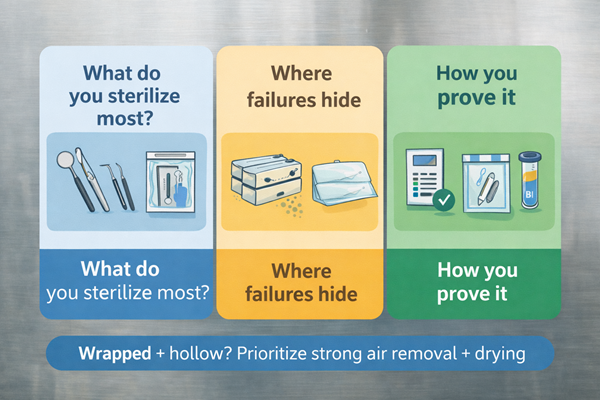
Load-to-autoclave mapping (decision table)
| Load type (what you actually run) | Typical dental workflow impact | Main risk mechanism | Capability you need | What proves it in routine use |
|---|---|---|---|---|
| Unwrapped solids (immediate use) | Fast turnarounds | Overloading and blocked steam paths | Stable process control + correct loading | Mechanical parameters + chemical indicators + weekly BI program CDC |
| Wrapped/pouched packs (for storage) | Sterile storage, scheduling | Wet packs; incomplete air removal; compromised dryness | Air removal + drying stability + load discipline | Internal CI in each package; mechanical records; weekly BI; release criteria for dryness CDC |
| Hollow / lumen items | High consequence if missed | Air pockets block steam penetration | Pre-vacuum air removal + validated cycles for lumens | Daily air-removal test when operated (pre-vac); routine leak/vac test; weekly BI OSAP |
| Mixed loads | Real-world “everything in one run” | Conflicting drying/heat transfer; shadowing | Defined load configurations + process control | Standardized loading patterns + monitoring + records CDC |
2) Class N / S / B: useful only if you translate it into load compatibility
EN 13060 class language matters only because it forces a load conversation: Class N typically covers unwrapped solid instruments (gravity displacement), Class S is load-specific (depends on validated load types), and Class B (pre-vacuum) is designed to handle wrapped and hollow loads. Source
If you routinely sterilize wrapped packs for storage and hollow/lumen items, prioritize validated air removal and drying performance—and ensure you can demonstrate it with routine testing and documentation. OSAP checklist
3) Sterility assurance is a monitoring + release system, not a temperature claim
CDC recommends monitoring sterilizer performance using a combination of: mechanical monitoring (time/temperature/pressure records), chemical indicators, and biological indicators (spore tests), with spore testing at least weekly. CDC
Audit-ready monitoring schedule
| Frequency | What you do | Why it matters |
|---|---|---|
| Every load | Review and document mechanical parameters (printout/log or manual record) | Confirms the cycle achieved programmed conditions CDC |
| Every package | Use a chemical indicator inside each package (plus external if internal isn’t visible) | Shows sterilant exposure/penetration to the pack CDC |
| Each day a pre-vac sterilizer is operated | Run an air-removal test (Bowie-Dick) | Verifies effective air removal / steam penetration capability OSAP |
| At least weekly (each sterilizer) | Run a biological indicator (spore test) with a matching control | Most accepted direct monitor; common minimum cadence CDC |
| After repairs, relocation, process changes, or a failed test | Quarantine loads as appropriate; investigate; correct; retest | Demonstrates corrective action and prevents unsafe release ADA |
The OSAP checklist aligns with this structure and explicitly calls out daily Bowie-Dick testing for pre-vac sterilizers and weekly biological testing. OSAP
4) Loading rules: the fastest way to fail a “good autoclave” is bad loading
Many “sterilization failures” in clinics are process failures: overloading, blocked steam pathways, poor pouch orientation, packs touching chamber walls, or incorrect load configurations. Internal checklist reference
Non-negotiable loading rules (clinic-practical) Guide
- Do not overload—steam must contact all surfaces.
- Keep pouches edge-on (rack them); avoid stacking pouches flat.
- Keep packs off chamber walls and allow space between items.
- Place heavier items below lighter packs/pouches to protect packaging and drying.
- Open hinged instruments (where IFU allows) so steam contacts joints.
- Only run validated load configurations for the selected cycle.

Loading discipline prevents false confidence—where the sterilizer meets parameters but the load doesn’t get sterilant contact. CDC
5) Drying and wet packs: release criteria, not opinions
For wrapped/pouched items intended for storage, the professional position is straightforward: wet packs should not be released for storage. Moisture compromises package integrity and increases contamination risk during handling and storage.
Release rule: If packs are wet or show condensation, treat the load as not acceptable for storage—investigate loading density, packaging choice, drying time, and equipment condition before reprocessing. Loading rules
6) Failure response: what to do when monitoring says “no”
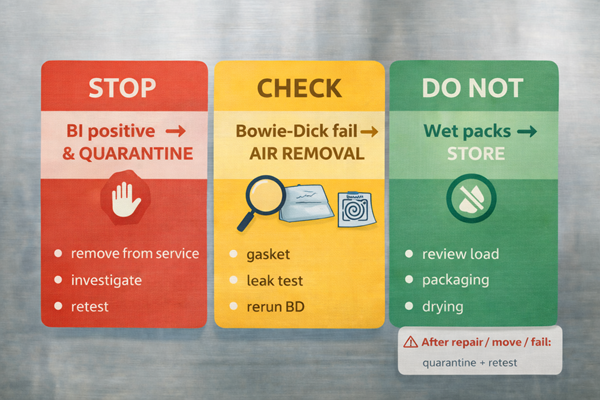
This is where your program stops sounding like marketing and starts sounding like QA: define actions tied to monitoring outcomes.
If a spore test is positive (BI failure)
Remove the sterilizer from service, review the process to rule out operator error, correct problems, and retest using biological, mechanical, and chemical indicators before returning to routine use. ADA
If a Bowie-Dick (air removal) test fails (pre-vac)
Treat it as an air removal performance problem: do not run production loads until resolved; investigate leaks, door gasket integrity, vacuum function, and cycle selection. STERIS
If chemical indicators fail in a pack
Do not release that pack; investigate cycle parameters, load configuration, and indicator placement; consider quarantining associated items in the same load based on your policy. CDC
For a structured, clinic-friendly root-cause workflow: start with cycle parameters and monitoring results, then inspect loading/packaging, then equipment and utilities. Root-cause checklist
7) Recordkeeping: the difference between “we ran it” and “we can prove it”
A defensible record ties who / when / what load / what cycle / what results together.
Minimum fields that make records useful:
- Sterilizer ID (and cycle/load number)
- Date/time
- Cycle type/program
- Mechanical parameters (or printout attachment)
- Chemical indicator results (per pack or per load policy)
- BI lot number + control + result (weekly, and after changes)
- Operator initials
- Exceptions and corrective actions
This documentation aligns with the “mechanical + chemical + biological” monitoring framework and makes audits survivable. CDC
8) Standards language
Modern moist-heat sterilization programs are built around validated cycles, routine control, and ongoing monitoring, consistent with how ISO 17665 frames steam sterilization processes. ISO 17665
For hospital SPD ecosystems, AAMI guidance is commonly used as a sterility assurance framework. AAMI ST79 (public copy)
9) Example configurations
This section should read like procurement guidance: load → capability → proof → fit.
Option A — Gravity tabletop steam sterilizer (for straightforward loads)
For clinics primarily processing unwrapped solids and straightforward workflows, a gravity tabletop sterilizer focuses on stable process control, safety protections, and basic drying support.
Example technical profile (JIBIMED TM-XB20J / TM-XB24J series):
- 20 L / 24 L chamber volumes with listed chamber dimensions
- 134°C working temperature; 0.22 MPa working pressure
- 105–134°C adjustable range; 0–60 min timer
- Stainless steel chamber; three trays; automatic cool air exhaust
- Water-lack protection; over-temperature and over-pressure protection
- Steam-water inner circulation described as “no steam discharge”; end-of-cycle beep reminder
Reference: TM-XB20J / TM-XB24J page
Professional positioning: Use it for validated loads that match the cycle design; if you need routine sterilization of wrapped packs and lumen items, prioritize pre-vac air removal and drying validation. Class overview
Option B — Class B tabletop pulse vacuum sterilizer (for wrapped + hollow/lumen + mixed loads)
For dental clinics processing wrapped packs and hollow/lumen instruments, Class B (pre-vac) units are chosen for air removal performance, steam penetration, and drying stability—supported by routine testing and documentation.
Example technical profile (JIBIMED TM-12DV / TM-20DV / TM-24DV):
- Standard Class B with three times vacuum and drying
- Vacuum data stated to reach -0.8 bar
- Built-in Bowie-Dick test and vacuum test functions
- Built-in mini printer for sterilization records
- 134°C working temperature; 0.22 MPa working pressure; adjustable 105–134°C; 0–99 min timer
Reference: Class B pulse vacuum page
Professional positioning: Connect features to your QA program—daily air-removal testing when operated (pre-vac), mechanical logging/printouts, internal chemical indicators in packs, and weekly biological monitoring. OSAP
FAQ
How often should we run spore tests?
At least weekly per sterilizer, using a matching control, with additional testing after changes/repairs; follow manufacturer IFU and local requirements. CDC
Do we need chemical indicators in every pack?
Use a chemical indicator inside every package; add an external indicator if the internal indicator is not visible. CDC
Is “flash / unwrapped sterilization” a good daily workflow tool?
No. CDC states it is not viable for routine use and should not be used for convenience, to save time, or to avoid buying more instrument sets. CDC
What’s the most common cause of sterilization failure in clinics?
Process errors: incorrect cycle selection, inadequate air removal, improper loading/packaging, and maintenance/utility issues—troubleshoot from monitoring results outward. Checklist
Why do we still need a Bowie-Dick test if we have a leak test?
They evaluate different performance aspects; daily air-removal testing is a common QA expectation for pre-vac systems when operated. OSAP
References
- CDC: Sterilization and Disinfection (Dental Settings) — https://www.cdc.gov/dental-infection-control/hcp/summary/sterilization-disinfection.html
- OSAP: Sterilization Monitoring & Storage Checklist — https://www.myads.org/assets/docs/resources/the-safest-dental-visit/osap-checklist-sterilization-monitoring-and-storage.pdf
- ISO 17665 overview — https://www.iso.org/standard/80271.html
- Tuttnauer: Types of Autoclave Sterilizers (Class N/S/B overview) — https://tuttnauer.com/knowledge-center/sterile-processing/autoclave/types-of-autoclave-sterilizers
- JIBIMED: Gravity Tabletop Steam Sterilizer — TM-XB20J/TM-XB24J)
- JIBIMED: Class B Pulse Vacuum Steam Sterilizer — TM-12DV/TM-20DV/TM-24DV


 English
English русский
русский Français
Français Español
Español bahasa Indonesia
bahasa Indonesia Deutsch
Deutsch عربى
عربى 中文简体
中文简体


















