Hydrogen Peroxide Plasma Sterilization: Principle, Pros & Limits
Posted by Admin | 19 Jan
Hydrogen peroxide low-temperature plasma sterilization is a fast, low-heat method for heat- and moisture-sensitive medical instruments, but it has strict limitations on lumen geometry, material compatibility, and dryness. In practice, it fills the gap for delicate endoscopic and precision devices that cannot tolerate high-temperature steam, while requiring disciplined loading, drying, and device selection to avoid failures or damage.

What H2O2 low-temperature plasma sterilization is
Low-temperature plasma sterilization uses hydrogen peroxide (H2O2) as the sterilant and converts it into a plasma state under controlled conditions. This process inactivates microorganisms on medical and surgical instruments—including bacteria, viruses, fungi, and spores—without exposing devices to the high heat and humidity of steam sterilization.
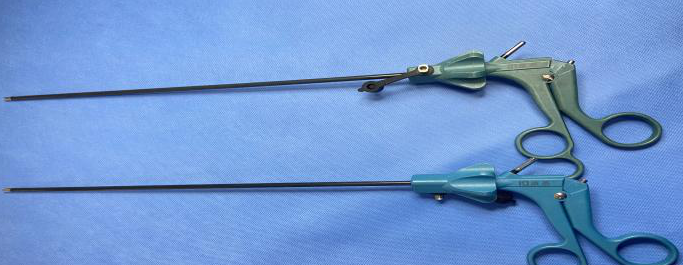
Because the operating conditions are typically around 45°C with very low humidity (about 10%RH), it is widely used for precision instruments where heat, moisture, or repeated thermal cycling may deform materials, damage seals, or reduce optical/electronic performance.
Sterilization mechanism: how plasma achieves microbial kill
The sterilization effect comes from the combined action of hydrogen peroxide and the plasma phase. During the cycle, hydrogen peroxide is introduced and then energized into a plasma, producing reactive species that cause oxidative damage to microbial components, leading to loss of viability.
- Oxidation-based inactivation: reactive species attack cell membranes, proteins, and nucleic acids, preventing replication and survival.
- Low heat and low moisture: reduces thermal and hydrolytic stress on delicate instruments compared with steam cycles.
- Decomposition endpoint: hydrogen peroxide breaks down after the process; the intended final residues are minimal when the cycle is correctly run.
Practical advantages in clinical workflows
The major operational value of H2O2 plasma sterilization is fast turnover for instruments that cannot undergo steam sterilization. This helps reduce instrument inventory pressure while maintaining surgical throughput.
- Rapid sterilization: short cycle time supports high case volumes and reduces turnaround delays.
- No long exhaust phase: H2O2 decomposes readily, enabling faster release of loads and improving circulation of high-value devices.
- Low-temperature operation: the process runs at about 45°C, protecting heat-sensitive materials and precision assemblies.
- Low-humidity environment: about 10%RH, reducing moisture-related risks in devices with electronics, optics, or adhesives.
- Simplified facility needs: typically requires only an electrical supply, with automated programs and no dedicated ventilation duct installation in many setups.
- Inventory and cost dynamics: faster turnover can reduce the number of instrument sets needed for a given surgical volume, supporting efficiency and cost control.
Key limitations and operational risks
The same mechanism that makes plasma effective also imposes constraints. The most common causes of unsuitable loads are limited penetration in complex lumens, oxidation sensitivity of materials, and strict requirements for dryness.
Penetration constraints in lumens and channels
Plasma sterilization has weaker penetration compared with saturated steam in certain configurations. Instruments with long, narrow internal channels must meet strict manufacturer limits for lumen length and diameter, otherwise the sterilant may not reach critical internal surfaces reliably.
Oxidation effects and instrument discoloration
Because the process relies on oxidative chemistry, repeated exposure can react with certain metals, polymers, coatings, dyes, and surface treatments. Over time, this may lead to color change on instruments or altered surface appearance, especially when materials are not fully compatible with repeated H2O2 plasma cycles.
High cost and strict preconditioning
- Higher per-cycle cost: consumables and equipment costs are typically higher than steam sterilization.
- Absolute dryness required: instruments must be completely dry before sterilization; moisture can impair the cycle and increase failure risk.
- No oiling: devices cannot be lubricated with oil prior to processing, because residues can interfere with sterilant action and compatibility.
Occupational safety considerations
Hydrogen peroxide has inherent toxicity at sufficient concentration, and the plasma process may involve ultraviolet radiation within the chamber. Safe operation depends on intact equipment, correct cycle selection, and adherence to manufacturer instructions for handling, loading, and maintenance.
What can be sterilized with H2O2 plasma
H2O2 plasma sterilizers can process a broad range of metal and non-metal devices, especially items that are not resistant to heat or moisture. Clinically, it is commonly used for minimally invasive surgical sets and precision endoscopic instruments.

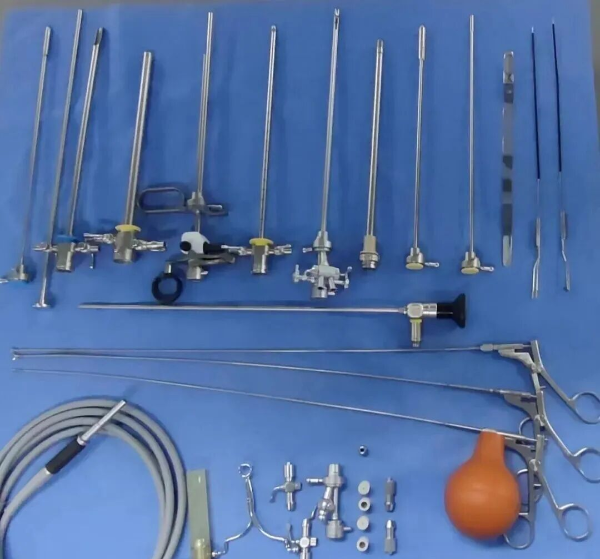
- Endoscopic and cavity instruments: arthroscopes, laparoscopes, otoscopes, electrosurgical scopes, ureteroscopes, cystoscopes, hysteroscopes, and related minimally invasive instruments, when they meet lumen specifications.
- Heat- and moisture-sensitive devices: instruments that would be damaged by high-temperature/high-humidity steam processing.
What must not be sterilized with H2O2 plasma
Load exclusions are critical because incompatibility can lead to sterilization failure, device damage, or safety risk. The following categories are generally not suitable unless the device manufacturer explicitly validates compatibility.
- Any instruments with blind-end or partially blind-end lumens where sterilant access is unreliable.
- Fiber-based items such as cotton cloth, paper, and gauze, including instrument boxes made of fibrous materials.
- Any liquids, oils, or powders.
- Devices that do not meet lumen length/diameter limits specified for the sterilizer and cycle.
- Single-use devices and disposable medical supplies when the manufacturer does not recommend reprocessing.
- Implants, unless the manufacturer explicitly recommends H2O2 plasma sterilization for the specific implant and system.
- Items containing nylon or nylon-derived materials on critical surfaces, where compatibility is uncertain or contraindicated.
- Any instrument or device that cannot tolerate vacuum phases, and any item labeled for use only with gravity-displacement steam sterilization.
A practical decision checklist for daily use
Use this checklist to decide whether an item is appropriate for H2O2 low-temperature plasma sterilization and to reduce failures or avoidable damage.
| Checkpoint | Pass criteria | If not met |
|---|---|---|
| Dryness | Completely dry, no residual moisture | Re-dry; do not run plasma cycle while wet |
| Lumen geometry | Meets sterilizer limits for length and diameter | Choose an alternative validated method |
| Material compatibility | Manufacturer validates repeated H2O2 plasma cycles | Risk of discoloration, oxidation, or performance change |
| Load type | No liquids, oils, powders, or fibrous materials | Exclude from plasma cycle and reclassify processing method |
| Vacuum tolerance | Device can tolerate vacuum stages | Risk of deformation/damage during evacuation |
Bottom line: H2O2 low-temperature plasma sterilization is a high-value solution for delicate instruments, but successful use depends on strict compatibility screening, fully dry loads, and adherence to lumen and vacuum constraints to avoid failures and instrument changes such as discoloration.


 English
English русский
русский Français
Français Español
Español Indonesia
Indonesia Deutsch
Deutsch عربى
عربى 中文简体
中文简体


















